Arrhenius
carbon dioxide concentration and climate.Arrhenius:
Arrhenius, Svante August (1859-1927) Swedish Chemist, Physicist Svante August Arrhenius was born in Vik (or Wijk), near Uppsala, Sweden, on February 19, 1859. He was the second son of Svante Gustav Arrhenius and Carolina Christina (nee Thunberg). Svante’s father was a surveyor and an administrator of his family’s estate at Vik. In 1860, a year after Arrhenius was born, his family moved to Uppsala, where his father became a supervisor at the university. He was reading by the age of three.
Arrhenius received his early education at the cathedral school in Uppsala, excelling in biology, physics, and mathematics. In 1876, he entered the University of Uppsala and studied physics, chemistry, and mathematics, receiving his B.S. two years later. While he continued graduate classes for three years in physics at Uppsala, his studies were not completed there. Instead, Arrhenius transferred to the Swedish Academy of Sciences in Stockholm in 1881 to work under Erick Edlund to conduct research in the field of electrical theory.
Electrical conductivity of dilute solutions:
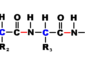
Arrhenius studied electrical conductivity of dilute solutions by passing electric current through a variety of solutions. His research determined that molecules in some of the substances split apart. Or dissociated from each other, into two or more ions when they were dissolved in a liquid. He found that while each intact molecule was electrically balanced, the split particles carried a small positive or negative electrical charge when dissolved in water. The charged atoms permitted the passage of electricity, and the electrical current directed the active components toward the electrodes. His thesis on the theory of ionic dissociation was barely accepted by the University of Uppsala in 1884, since the faculty believed that oppositely charged particles could not coexist in solution. He received a grade that prohibited him from being able to teach.
Arrhenius published his theories (“Investigations on the Galvanic Conductivity of Electrolytes”). And sent copies of his thesis to a number of leading European scientists. Russian-German chemist Wilhelm Ost- wald, was impressed and visited him in Uppsala. Offering him a teaching position, which he declined. However, Ostwald’s support was enough for Uppsala to give him a lecturing position, which he kept for two years.
Traveling scholarship:
The Stockholm Academy of Sciences awarded Arrhenius a traveling scholarship in 1886. As a result, he worked with Ostwald in Riga with physicist Friedrich Kohlrausch at the University of Wurzburg. Physicist Ludwig Boltzmann at the University of Graz. And with chemist Jacobus Van’t Hoff at the University of Amsterdam. In 1889, he formulated his rate equation that is used for many chemical transformations and processes, in which the rate is exponentially related to temperature, known as the “Arrhenius equation.”
He returned to Stockholm in 1891 and became a lecturer in physics at Stockholm’s Hogskola (high school) and was appointed physics professor in 1895 and rector in 1897. Arrhenius married Sofia Rudbeck in 1894 and had one son. The marriage lasted a short two years. Arrhenius continued his work on electrolytic dissociation and added the study of osmotic pressure.
Carbon dioxide concentration and climate:
In 1896, he made the first quantitative link between changes in carbon dioxide concentration and climate. He calculated the absorption coefficients of carbon dioxide and water based on the emission spectrum of the moon. And he also calculated the amount of total heat absorption and corresponding temperature change in the atmosphere for various concentrations of carbon dioxide. His prediction of a doubling of carbon dioxide from a temperate rise of 5-6°C is close to modern predictions. He predicted that increasing reliance on fossil fuel combustion to drive the world’s increasing industrialization would. In the end, lead to increases in the concentration of CO2 in the atmosphere, thereby giving rise to a warming of the Earth.
In 1900, he published his Textbook of Theoretical Electrochemistry. And in 1901 he and others confirmed the Scottish physicist James Clerk Maxwell’s hypothesis that cosmic radiation exerts pressure on particles. Arrhenius went on to use this phenomenon in an effort to explain the aurora borealis and solar corona. He supported the Norwegian physicist Kristian Birkeland’s explanation of the origin of auroras that he proposed in 1896. Arrhenius also suggested that radiation pressure could carry spores and other living seeds through space and believed that life on earth was brought here under those conditions. He likewise believed that spores might have populated many other planets, resulting in life throughout the universe.
In 1902, he received the Davy Medal of the Royal Society and proposed a theory of immunology. The following year he was awarded the Nobel Prize for chemistry for his work that originally had been perceived as improbable by his Uppsala professors. He also published his Textbook of Cosmic Physics.
The Nobel Institute of Physical Chemistry:
Arrhenius became director of the Nobel Institute of Physical Chemistry in Stockholm in 1905 (a post he held until a few months before his death). He married Maria Johansson and had one son and two daughters. The following year he also had time to publish three books, Theories of Chemistry, Immunochemistry, and Worlds in the Making.
He was elected a foreign member of the Royal Society in 1911, the same year he received the Willard Gibbs Medal of the American Chemical Society. Three years later he was awarded the Faraday Medal of the British Chemical Society. He was also a member of the Swedish Academy of Sciences and the German Chemical Society.
During the latter part of his life his interests included the chemistry of living matter and astrophysics, especially the origins and fate of stars and planets. He continued to write books such as Smallpox and Its Combating (1913), Destiny of the Stars (1915), Quantitative
Laws in Biological Chemistry (1915), and Chemistry and Modern Life (1919). He also received honorary degrees from the universities of Birmingham, Edinburgh, Heidelberg, and Leipzig and from Oxford and Cambridge Universities. He died in Stockholm on October 2, 1927, after a brief illness, and is buried at Uppsala.