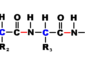
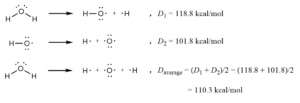Bond energy
bond energy (link dissociation force) Atoms in a molecule are held together by covalent bonds. To break these kind of linked atoms need bond energy. The source of energy to break the bonds can be in the form of heat, electricity, or mechanical means. This attractive force is the quantity of force that must be absorbed to break a particular kind of chemical bond. It is equal to the quantity of force the bond releases when it forms. It can also be defined as the amount of power necessary to break one mole of bonds of a given kind (in gas phase).