Alpha helix
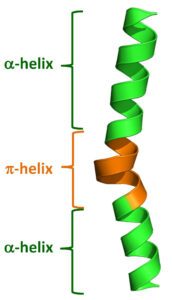
alpha helix (α helix) Most proteins contain one or more stretches of amino acids that take on a particular shape in three-dimensional space. The most common forms are α helix and beta sheet.
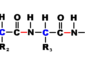
α helix is spiral shaped, constituting one form of the secondary structure of proteins, arising from a specific hydrogen-bonding structure; the carbonyl group (—C=O) of each peptide bond extends parallel to the axis of the helix and points directly at the -N-H group of the peptide bond four amino acids below it in the helix. A hydrogen bond forms between them
[-N-H………. O=C-] and plays a role in stabilizing the helix conformation. It is right-handed and twists clockwise, like a corkscrew, and makes a complete turn every 3.6 amino acids. The distance between two turns is 0.54 nm. However, it can also be left-handed. Most enzymes contain sections of alpha helix.
The alpha helix was discovered by Linus Pauling in 1948.