Amino acid
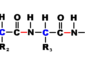
amino acid An organic molecule possessing both acidic carboxylic acid (-COOH) and basic amino (-NH2) groups. Which are attached to the same tetrahedral carbon atom.
Amino acids are the principal building blocks of proteins and enzymes. They are incorporated into
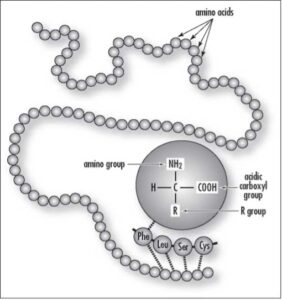
| Amino acids comprise a group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the “building blocks” of proteins. (Courtesy of Darryl Leja, NHGRI, National Institutes of Health) |
proteins by transfer RNA according to the genetic code. While messenger RNA is being decoded by ribosomes. The amino acid content dictates the spatial and biochemical properties of the protein or enzyme during and after the final assembly of a protein.
Amino acids have an average molecular weight of about 135 daltons. While more than 50 have been discovered, 20 are essential for making proteins, long chains of bonded amino acids.
Some naturally occurring amino acids are alanine, arginine, asparagine, aspartic acid, cysteine, glutamine, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine, and valine.
hydrophobic or hydrophilic
The two classes of amino acids that exist are based on whether the R-group is hydrophobic or hydrophilic. Hydrophobic or nonpolar amino acids tend to repel the aqueous environment and are located mostly in the interior of proteins. They do not ionize or participate in the formation of hydrogen bonds.
On the other hand, the hydrophilic or polar amino acids tend to interact with the aqueous environment.And are usually involved in the formation of hydrogen bonds. Usually found on the exterior surfaces of proteins or in their reactive centers. It is for this reason that certain amino acid R-groups allow enzyme reactions to occur.
The hydrophilic amino acids can be further subdivided into polar with no charge. Polar with negatively charged side chains (acidic), and polar with positively charged side chains (basic).
While all amino acids share some structural similarities. It is the side groups, or “R”-groups as they are called, that make the various amino acids chemically and physically different from each other so that they react differently with the environment. These groupings, found among the 20 naturally occurring amino acids.
Naturally occurring amino acids
naturally occurring amino acids are ionic (aspartic acid, arginine, glutamic acid, lysine, and histidine), polar (asparagine, serine, threonine, cysteine, tyrosine, and glutamine), and nonpolar amino acids (alanine, glycine, valine, leucine, isoleucine, methionine, phenylalanine, tryptophan, and proline).
Amino acids are also referred to as amphoteric, meaning they can react with both acids and alkali. Which makes them effective buffers in biological systems. A buffer is a solution where the pH usually stays constant when an acid or base is added.
In 1986 scientists found a 21st amino acid called “seleno- cysteine”. In 2002 two teams of researchers from Ohio State University identified the 22nd genetically encoded amino acid, called pyrrolysine. A discovery that is the biological equivalent of physicists. Finding a new fundamental particle or chemists discovering a new element.
Amino acid supplements are widely used in exercise and dietary programs.