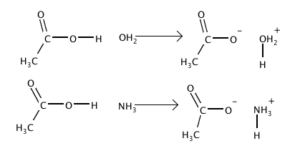
Base
base A substance that reduces the hydrogen ion concentration in a solution. A base has less free hydrogen ions (H+) than hydroxyl ions (OH–) and has a pH of more than 7 on a scale of 0-14. A base is created when positively charged ions (base cations) such as magnesium, sodium, potassium, and calcium increase the pH
of water when released to solution. They have a slippery feel in water and a bitter taste. A base will turn red litmus paper blue (acids turn blue litmus red). The three types of bases are: Arrhenius, any chemical that increases the number of free hydroxide ions (OH–) when added to a water-based solution; Bronsted or Bronsted-Lowry, any chemical that acts as a proton acceptor in a chemical reaction; and Lewis, any chemical that donates two electrons to form a covalent bond during a chemical reaction. Bases are also known as alkali or alkaline substances, and when added to acids they form salts. Some common examples of bases are soap, ammonia, and lye.